Is the atom really indivisible?
“Atom” comes from the ancient Greek for “indivisible”, a highly optimistic assumption that nothing smaller existed. As summed up in this strange nursery rhyme there is always something underneath: “Big fleas have little fleas, upon their backs to bite ’em, and little fleas have lesser fleas, and so, ad infinitum.”
100 years ago Neils Bohr was awarded the Nobel Prize for his model of the atom, still taught in schools today. Scientists have had all those years to search for the “lesser fleas” and root out more theories of how our universe is built and reacts all around us. Let’s start at the beginning of the idea that everything is made up of something smaller.
The first ideas of the atom were from ancient philosophers, such as Democritus, in around 400 BC. Millennia ahead of his time the idea that there were tiny, invisible balls making up everything in the world was to most people: creep, poppycock and insane. Yes, some would say it is still all of those things, yet come the 19th century the facts started to become indisputable.
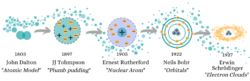
John Dalton was the first to announce an “Atomic Theory” in 1803. He told the world that; 1) an atom was the smallest unit of matter 2) each element had its own atom (distinguished by their weight) and 3) that when atoms interacted compounds were formed. Correct on most counts, Dalton revealed a great new field of physics, yet got a bit ahead of himself in using Democritus’ naming of “Atoms”, as it was soon discovered that these “indivisibles” were in fact made up of other particles.
Here enters the electron! Crazy little whizzing chaos balls. 90 years after Dalton, the idea of the atom had become common when JJ Thompson smashed the boundary of atoms being the most fundamental particle. With this revelation of a tiny negatively charged particle found within the atom, he had to re-model what an atom is and came up with “The Plumb Pudding Model”. This found electrons swimming in a sea of positive charge within the atom (much in the way raisins hide within a plumb pudding).
The dawn of the 20th century brings scientists bubbling with ideas for the new era. One such man was Rutherford who, in 1905 cut open the plumb pudding and pulled out all the raisins. He proved that the atom was segregated into compartments, a positive nucleus surrounded by empty space where electrons are found. (I and hungry physics students everywhere will be forever thankful to him for an atomic model that isn’t named after cake.)
Here is where Niels Bohr entered the scene as Rutherford’s student. His 1922 Nobel prize was for discovering that the atom was even further organised than in Rutherford’s model. Electrons weren’t free roaming but contained to set orbits around the nucleus which they could jump between if given energy, or fall from using energy. This model makes organised people happy – such a shame that just around the corner quantum mechanics was lurking to mess it all up.
Not even five years after this Nobel Prize, Erwin Schrödinger revealed “Wave Theory”. Rather than circling like tiny planets around the nucleus, the particles become waves filling the 3D space. However, where it gets really weird is that when we look at electrons they act as particles. So Schrödinger mapped where he found electrons to create a new model of electron clouds.
1927, just one year after Schrödinger’s reveal, Heisenberg made this tiny world even stranger. He tells us that not only does an electron stop acting as a wave when we look at it, but we also cannot know one electron’s speed at the same time as we know its location. We can’t watch it move and see how fast it’s going, we only see the freeze frame. Vice versa, if we know how fast it’s going we have no idea where it is. Aptly this is called the “Uncertainty Principle.”
All this seems to get a little skipped over in physics at school as we jump straight to the neutron discovery which wasn’t until 1931. From here, even more subatomic particles, as they are now known, were discovered, from electrons to quarks and most recently bosons. The atom, which was supposed to be the smallest unit of matter has been split into almost 20 particles with more to be discovered.
This field of physics has expanded our understanding of how things interact on a universal scale, from explaining fireworks, to firing subatomic particles at biological proteins to discover their shapes, and the current attempt to recreate nuclear fusion for sustainable energy. All these advances are based on our understanding of the atom. So even if it sounds gross, from observing the “smaller fleas” we can find yet smaller fleas and use that knowledge to improve the world around us.
Comments