Are drug-producing bacteria just beyond the horizon?
Thanks to new research, we are a step closer to programming bacteria to synthesise drugs; a huge advancement in medicine.
A team led by University of Warwick’s Integrative Synthetic Biology Centre and the Faculty of Health and Medical Sciences at the University of Surrey, has had a recent breakthrough. They have developed a unique system which dynamically allocates essential cellular resources inside cells engineered using synthetic biology techniques; advancing the potential of synthetically programming cells to combat disease and produce new drugs.
They have developed a unique system which dynamically allocates essential cellular resources inside cells
Synthetic biology has been heralded as the beginnings of the next Industrial Revolution. It is an interdisciplinary field which applies principles of engineering to biological systems, in order to design new biological systems or redesign existing ones. This brings together geneticists, chemists, computer engineers and more to develop tools to enhance our current understanding of biology. The field offers great opportunities to create processes and products, such as these drugs, to help address many of the challenges that face our world today; from health and food security, to energy and biotechnology.
Research in the field of synthetic biology often focusses on building synthetic biological circuits in cells, from a series of biological parts to enhance a cell’s performance and manipulate its functions. These circuits are similar to common electrical circuits but have more variables and complications to consider. Currently, a complication is that many of the designed circuits initially fail upon implementation into the cell due to unforeseen interactions between the host and the synthetic circuit. Often, there is competition for shared cellular resources, such as ribosomes (microscopic ‘factories’ inside cells, responsible for building proteins), of which there are a finite number in a cell. The distribution of ribosomes is often static; either the host cell gets most of the resources, or the circuitry does, which can cause cell death or circuitry failure.
Synthetic biology has been heralded as the beginnings of the next industrial revolution
Previously this has been combatted by complex redesign of ribosomes or incorporating negative feedback loops into the circuit, however this research approaches the problem in a different (and more efficient) way, bypassing the need for extensive redesign. They have found a way to distribute the ribosomes dynamically, courtesy of engineering’s feedback control loop principal, they developed a unique system that allocates ribosomes to either the circuit or the cell depending on which one needs more ribosomes at that particular moment in time.
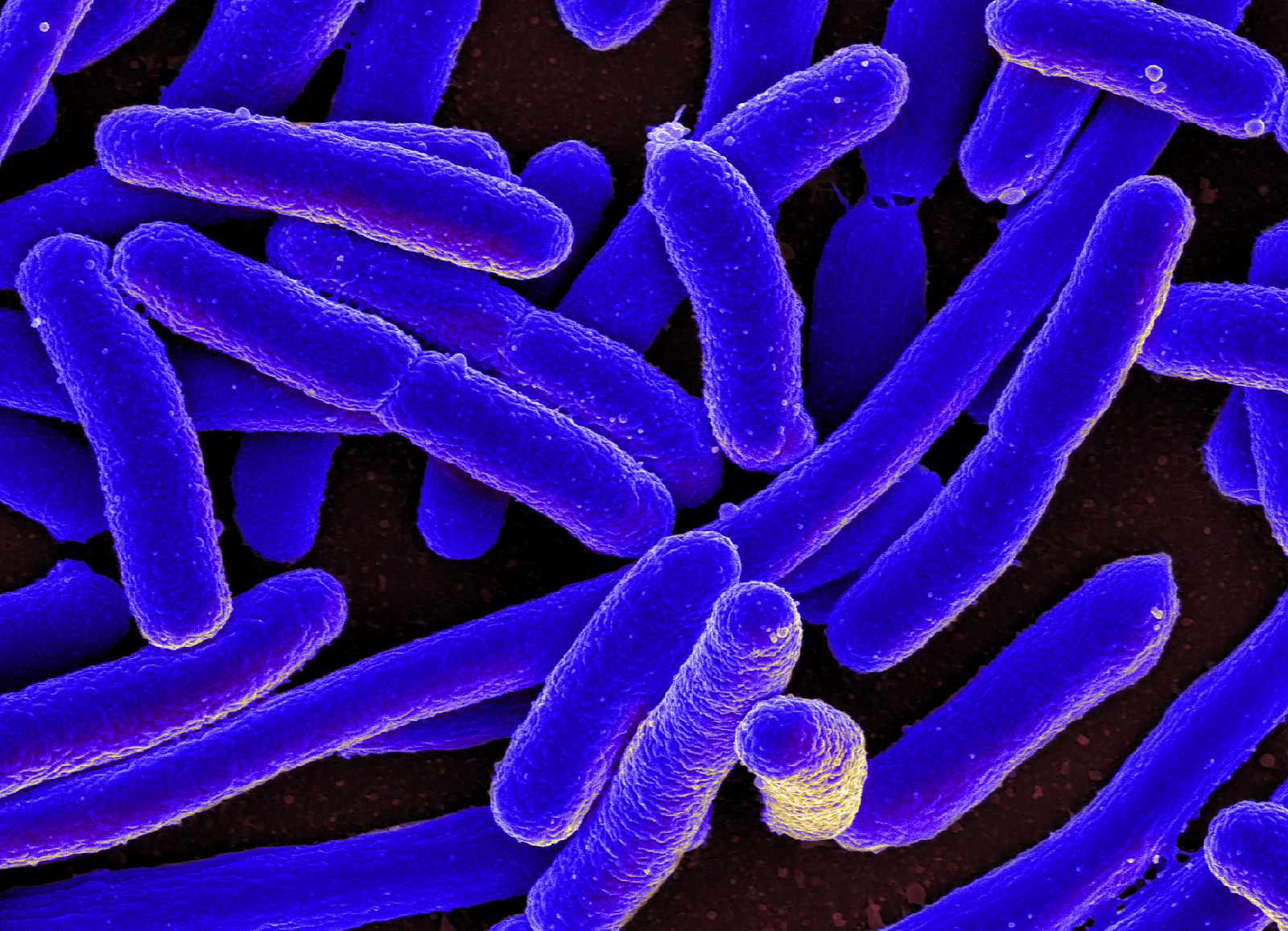
These findings have real implications, as stated in the paper published in Nature Communications in February, they can massively facilitate the “implementation of synthetic gene circuits in future biotechnological applications”, unlocking the various exciting possibilities for the future of healthcare and pharmaceuticals. One potential application is the exciting venture into drug-producing bacteria. Bacteria are single-celled organisms which have been used in bioengineering for decades as they are relatively easy to work with, and this discovery will make it even easier to add synthetic circuitry to the organisms, enabling the bacteria to be used as a “factory” to produce antibiotics and other valuable drugs, like antimalarials.
This discovery will make it even easier to add synthetic circuitry to the organisms
This is an exciting time for the field synthetic biology, which according to Professor Declan Bates “is about making cells easier to engineer so that we can address many of the most important challenges facing us today”. This development has massive potential, from “manufacturing new drugs and therapies to finding new biofuels and materials”. This finding has helped propel the discipline along even further to its target which was said by Dr José Jiménez from University of Surrey to be “understand[ing] biology itself”.
Comments